Atomic Structure
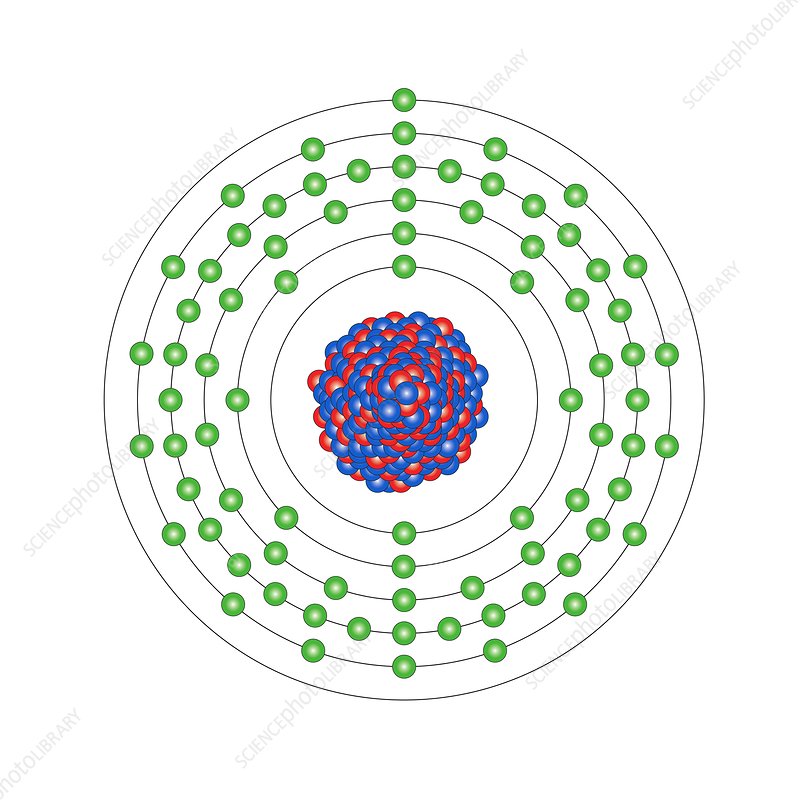
The atomic number of gold is 79. It has two different isotopes: 198 Au and 197 Au. 197 Au has 79 protons and eletorons, and 112 neutrons. 198 Au has 79 protons and eletorns, and 113 neutrons. It is in group 11, period 6 on the periodic table. The mass number is 196.967.
Appearance and Physical Attributes
Gold is a relatively soft metal awith distinct yellowish color. It is unreactive, although it dissolves in agua regia. It has a density of 19.3 g/cm3. It melts at 1064 C and boils at 2836 C. Gold is mainly used as currency or as jewelry.
History of Gold
Gold was one of the first metals or even elements to be discovered and used. It's been known since prehistory times. It was first found in big nuggets that were in streams and caves. The first known people to have reallyl used gold were the ancient Egyptions who used it in a tomb for their King Tutankamen. Nowadays gold is mostly just used as currency and jewelry, but its soft, malleable structure makes it great for art.
Additional Facts About Gold
- Gold has a half life of 2.69f d
- The symbol Au comes from the latin word Aurum which means gold.
- The top 3 producers of gold are China, Australia, and USA
- Gold has a medium supply risk
- Gold is non-toxic
- About 1500 tons of gold are mined per year
- Gold was discovered at approx. 3000 BC